Transit (Transportation ) stability study is very important for Pharmaceutical Industries to export the medicines to other countries.
After excursion a complete stability study data is required up to product Shelf life at marketed condition of subject Zone/Countries.
Generally excursion has been given as below before charging in stability chamber at marketed condition:
| At 50°C/75% RH or more than 50°C/75% RH | At least 03 days |
| At -20°C or less than -20°C | At least 03 day or up to 10 days. |
After above excursion charge the sample at marketed condition up to product shelf life and perform the stability study as per routine stability study program.
During medicine transport, a Data logger is kept along with medicine and after reaching its destination the record of temperature and humidity has been checked. If any excursion found beyond our available Transportation stability study data then medicine should not be distributed in market.
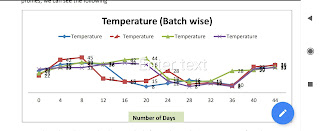
Figure Example of a temperature profile graph for four shipments as below:
As per above data shipment has been reached after 44 days and minimum temp. is 8°C and Maximum temperature is 45°C.
If our excursion study data is passed up to shelf life , then above batch can be distribute in market.
Introduction :
Technical supplement has been written to amplify the recommendations given in Section 6.8.3 and 6.8.4 of WHO Technical Report Series No. 961, 2011, Annex 9: Model guidance for the storage and transport of time- and temperature-sensitive pharmaceutical products.
Requirements:
Regulators increasingly require pharmaceutical transport operators to document their shipping practices in a manner which shows that they fully understand their transport process and are able to maintain control over it. As part of the process of validating these practices, the performance qualification of shipping containers and refrigerated vehicles should be based on transport route profile(s) which reflect the real distribution environment in a statistically robust manner. Consequently, the initial route profiling exercise should be carried out before actual products are distributed.
Objectives:
a. A comprehensive and systematic approach to monitoring temperatures in a distribution system;
b. A protocol for temperature data collection;
c. A method for converting these data into a representative transport route profile with multiple confidence levels;
d. A simple method for estimating the performance of prequalified containers, laboratory tested at constant ambient temperature(s), against an ambient temperature profile.
e. A method for determining representative distribution temperatures for the qualification of shipping system
Nice blog sir 👍
ReplyDelete